Artificial Fertilizer
Title
Subject
Description
Without fertilizer nearly half of the world’s population would be without food. Synthetic fertilizer or the synthesis of ammonia has been highly regarded as one of the most important technological inventions of the twentieth century due to its ability to feed our ever growing population (Brassley 2002, 149). Specifically, the development of artificial fertilizer allowed for the mass production of crops (Marx 1974, 132). Robert Paskach photographed this fertilizing machine taken in 1970. (Figure I) This photograph speaks to two important historical transitions. First, the growth of the global population and the concurrent need to increase agricultural yields and second, the environmental costs of input intensive fertilizer use.
Nebraska is an ideal place to explore the global transition to artificial fertilizer use on a smaller scale. It is a largely agricultural state, and even urbanized areas like Omaha are part of the story. Many Nebraskans rely on farming or agriculture as a living, and those citizens then contribute to the well-being of the state. According to a 2012 study about the impact of agriculture on Nebraska in 2010, agriculture accounted for about 24% of the state’s workforce, and accounted for 40% of the state’s economic output. According to the same study, agriculture made up $22.6 billion of Gross State Product, which is 26.9% of the total Gross State Product for 2010 (Thompson, Johnson, Giri, 2012). Due to the importance of in Nebraska, farmers have always been looking for the best way to get the most out their crops. Nebraskan farmers employed fertilizers long before the advent of the Haber-Bosch process. They relied extensively on manuring, for instance, to increase soil fertility.
Farmers have been using manure for thousands of years in their agricultural practices. These early farmers noticed increased crop growth in areas where there was a large amount of dung accumulation left from animals. There is evidence of ancient civilizations, such as the Egyptians and Romans, using minerals and other sources nutrients for their soil, but the main source was for thousands of years was manure from animals (Herbert, 2015).
Farmers also relied on other substances besides manure to provide nutrients to the soil and their crops as time progressed. During the 18th century it was common for ground-up bones to be used to provide nutrients. In the United States, bones were gathered from livestock packing houses, and by the bison that were slaughtered on the prairies (Hergert, 2015). Historian Geoff Cunfer explains just how important adding nutrients to the soil was to farmers in the Great Plains in 1886. “Farmers make their living,” he explains, “by slightly altering nature to achieve human ends...In short, the farmers’ métier has everything to do with flows of energy through ecosystems, fluxes of hydrology, and the invisible transference of nitrogen from air to soil and back again." (Cunfer, 2004, 539)
By the late nineteenth-century, fears of a soil nutrient crisis prompted Robert Furnas, the first president of the Board of Agriculture in Nebraska, to push for changes in farming practices. In his third annual report in 1871, he suggested farmers “reduce the area of superficial surface cultivated, and increase the producing capabilities of the soil, by deep and thorough cultivation...and applying fertilizers adapted the wants of the crop designed to grow.” (Sweedlun 1940, 56). Manuring had limits, however, and the industrial, commercial development of artificial fertilizers opened up a new frontier in fertilizer use.
Justus von Liebig, a German chemist in the late 19th century, discovered the three chemical elements needed for plant growth: nitrogen, phosphorus, and potassium. The least available of these nutrients determines the potential for crop growth, Liebig also discovered. The findings of his research led him to develop the first nitrogen-based fertilizer, and he became known as the, “Father of the Fertilizer Industry.” (Herbert, 2015) As the fertilizer industry was changing, it prompted other scientists to find new ways to create the nutrients needed to promote crop growth.
In 1909, German chemists Fritz Haber and Carl Bosch made a historical breakthrough that enabled them to fixate nitrogen from air. This process required high temperatures and pressure along with the aid of a catalyst, nitrogen gas (N2) in combination with three molecules of hydrogen gas (H2) to produce two molecules of ammonia (Hughes 1969, 108). N2+ 3 H2→ 2 NH3 is widely referred to as the Haber-Bosch process. By removing the transformed liquid ammonia from the system, Haber and Bosch allowed for the continuous production of ammonia. They ultimately commercialized this process and by the mid-twentieth century, farmers in the US increasingly relied on corporations to improve soil fertility. This was a significant contribution to the agricultural industry because it enabled our population to grow from 1.6 billion people to roughly 6 billion and without this chemical reaction nearly 3 billion people would be without food (Smil 1997, 76).
After World War II fertilizer use increased dramatically as it became easier to manufacture, and there was a greater need for food. In the U.S. 10 plants produced ammonia during the war, and they were all located in the middle of country, and near natural gas pipelines. This created easy access to creating nitrogen from the gas. Once the war ended food supplies need to be restored in the United States and Europe. This created a surge of research into agriculture developments such as new and improved hybrids, equipment design and performance, and basic guidelines for crop nutrition and fertilizer requirements. After World War II about 2 million tons of chemical fertilizer was used each year, that increased to over 7 million per year by 1960, and increasing further to over 20 million tons in 2014. (Hergert, 2015)
One such corporation was Scotts, which supplied farmers near Omaha with artificially-produced Turf Builder Fertilizer (Figure II). Scotts was among the first to recognize that the nutritional needs of crops required a specifically designed fertilizer. Its composition includes 32% total nitrogen as well as potassium sulfate, sulfur, and iron. Although these ingredients are biodegradable, if applied in high enough volumes it contaminates local water supplies. This problem affected Scotts, as well as the many other companies supplying fertilizers for American farmers. Today, Scotts focuses on lawn care, but they also sell specialty fertilizers that can be used for many different types of crops such as tomatoes, citrus fruits, and avocados. Artificial fertilizer thus demonstrates a power of art in its allowance for the increased cultivation of a variety crops all with differential needs.
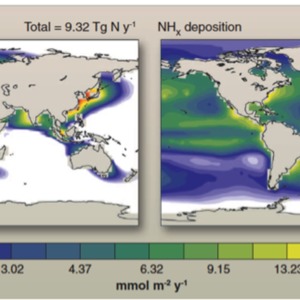
Although the Haber-Bosch process has greatly contributed to the expansion of the human population, it has come with considerable impacts on the environment. A major consequence stems from the fact that a significant amount of nitrogen-based fertilizer does not end up assimilated by crops. Excess ammonia runs off into areas where nitrification occurs leading to the contamination of coastal waters and a disruption to the natural nitrogen cycle. Excess nutrient availability in the water allows for an exponential level of replication and reproduction that exceeds the carrying capacity of the ecosystem (Borchardt 1969, 273). For example, algae draw from this increased nutrient availability and flourish. Their death and decomposition consumes oxygen, producing a hypoxic environment along the shoreline (Richard et. al 2000, 361). Low oxygen levels in the water negatively affect the physiology of higher aquatic animals, creating marine dead zones. Further research has shown that coastal hypoxia is actually expanding into open ocean waters. (Doney 2010, 1514). (Figure III) Fertilizer runoff, as well as a host of other factors such as fossil fuel combustion, expand the duration, intensity, and extent of coastal hypoxia leading to a reduction in overall marine biodiversity.
Nitrogen runoff and eutrophication demonstrate multiple clear connections to the Anthropocene. Some scholars argue that anthropogenic soils (Anthrosols) hold evidence (golden spikes) for the Anthropocene (Certini et. al, 1270). Anthrosols have been modified by human activity, including the addition of fertilizer. As an industrially-produced, commercial product, our object also highlights connections to the growth of international capitalism as well as the industrial revolution (Taylor 1961, 343). The need for synthetic ammonia responded to local and global population growth. The inventions of Fritz Haber and Carl Bosch sustained such a population size and humans were able to once again shape the world to fit our needs (Sundberg, Haber-Bosch & the Guano Lords).
Even with their continued use, many farmers, scientists, and policymakers have seen the consequences that fertilizers have had on the environment. The push for sustainable agricultural practices has pushed farmers to begin experimenting with older model of crop and livestock agriculture, hearkening back to pre-industrial practices employed before the development of chemical fertilizers.Farmers are seeking to go back to this process as a way of reducing reliance on fossil fuels, and minimizing their use of increasingly expensive fertilizer that is also adding to water pollution (Davis, 2012). One example is the using perennial crops to address these problems. Perennial crops don’t need to be reseeded every year, and have many benefits to the environment that can be seen from them being used already for thousands of years. These types of crops can increase nutrient retention for ecosystems, contribute to climate change adaptation, and they help ensure food and water security over a longer period of time (Land Institute, 2018) We know now the impact that chemical fertilizer has had on the environment, and how that could get worse if the current use of fertilizer is sustained. Humans can have a create negative impact on the environment when we are not thinking about the consequences of our actions, but humans can also create of positive impact on the environment when we try to learn for our mistakes.
Creator
Abaigh Plummer
Source
Ansari, Abid A. Eutrophication: Causes, Consequences and Control. Vol. 2. Dordrecht: Springer, 2014.
Borchardt, J.A. "EUTROPHICATION—CAUSES AND EFFECTS." Journal (American Water Works Association) 61, no. 6 (1969): 272-75. http://www.jstor.org.cuhsl.creighton.edu/stable/41266097.
Brassley, Paul. 2002. The Agricultural History Review 50 (1): 149-51. http://www.jstor.org.cuhsl.creighton.edu/stable/40275796
Certini, Giacomo, and Riccardo Scalenghe. “Anthropogenic Soils Are the Golden Spikes for the Anthropocene.” The Holocene 21, no. 8 (December 2011): 1269–74. doi:10.1177/0959683611408454.
Cunfer, Geoff. "Manure Matters on the Great Plains Frontier." Journal of Interdisciplinary History34, no. 4 (2004): 539-67. doi:10.1162/002219504773512534.
Davis, Adam S., Jason D. Hill, Craig A. Chase, Ann M. Johanns, and Matt Liebman. "Increasing Cropping System Diversity Balances Productivity, Profitability and Environmental Health." PLoS ONE7, no. 10 (2012). doi:10.1371/journal.pone.0047149.
Doney, Scott C. “The Growing Human Footprint on Coastal and Open-Ocean Biogeochemistry.” American Association for the Advancement of Science 328, no. 5985 (June 2010): 1512-1516.
doi:10.1126/science.1185198
Eutrophication: Causes, Consequences, Correctives. Washington, D.C.: Printing and Publishing Office, 1969.
Golding, Edward. "A History of Technology and Environment." 2016. doi:10.4324/9781315542959.
Hatfield, Jerry L., and Ronald F. Follett. Nitrogen in the Environment Sources, Problems, and Management. Academic Press, 2008.
Hergert, Gary, Rex Nielsen, and Jim Margheim. "Fertilizer History P1-P3." CropWatch. April 10, 2015. https://cropwatch.unl.edu/fertilizer-history-p3.
Hughes, Thomas Parke. "Technological Momentum in History: Hydrogenation in Germany 1898-1933." Past & Present, no. 44 (1969): 106-32. http://www.jstor.org.cuhsl.creighton.edu/stable/649734.
Madden, Patrick. "Low-Input/Sustainable Agricultural Research and Education: Challenges to the Agricultural Economics Profession." American Journal of Agricultural Economics 70, no. 5 (1988): 1167-172. http://www.jstor.org.cuhsl.creighton.edu/stable/1241757.
Marx, Jean L. "Nitrogen Fixation: Research Efforts Intensify." Science 185, no. 4146 (1974): 132-36. http://www.jstor.org.cuhsl.creighton.edu/stable/1738586.
McNeill, J. R., and Erin Stewart Mauldin. A Companion to Global Environmental History. Chichester: Wiley-Blackwell, 2015.
Perennial Grain Crop Development. (n.d.). Retrieved from https://landinstitute.org/our-work/perennial-crops/
Reinhardt, Claudia, and Bill Ganzel. "Fertilizers." Living History Farm. 2003. Accessed November 26, 2018. https://livinghistoryfarm.org/farminginthe30s/crops_08.html.
Richard A. Voyer, Carol Pesch, Jonathan Garber, Jane Copeland, and Randy Comeleo. "New Bedford, Massachusetts: A Story of Urbanization and Ecological Connections." Environmental History 5, no. 3 (2000): 352-77. http://www.jstor.org.cuhsl.creighton.edu/stable/3985481.
Shumway, Sandra E., JoAnn M. Burkholder, and Steve L. Morton. Harmful Algal Blooms: A Compendium Desk Reference. Hoboken, NJ: John Wiley & Sons, 2018.
Smil, Vaclav. "Global Population and the Nitrogen Cycle." Scientific American 277, no. 1 (1997): 76-81. http://www.jstor.org.cuhsl.creighton.edu/stable/24995834.
Sundberg, Adam. “Haber-Bosch & the Guano Lords.” Prezi Presentation. https://prezi.com/xjajurk_osco/haber-bosch-the-guano-lords/?utm_campaign=share&utm_medium=copy
Sweedlun, Verne S. A History of the Evolution of Agriculture in Nebraska, 1870-1930. Lincoln, NE, 1940.
Taylor, James. "THE MODERN CHEMICAL INDUSTRY IN GREAT BRITAIN." Journal of the Royal Society of Arts 109, no. 5057 (1961): 339-81. http://www.jstor.org.cuhsl.creighton.edu/stable/41366886.
Thompson, E., Johnson, B., & Giri, A. (2012). The 2010 Economic Impact of the Nebraska Agricultural Production Complex [Abstract]. Department of Agricultural Economics,(192). Retrieved from https://agecon.unl.edu/research/nebraska-ag-economic-impact.pdf.Collection
Citation
Embed
Copy the code below into your web page